India revokes patent of Novartis’ cardiac drug

NEW DELHI: The Indian Patent Office (IPO) has revoked the patent on Novartis’ cardiac blockbuster drug Vymada on grounds of lack of novelty, potentially paving the way for affordable generics to enter the market.The popular therapy has been in the spotlight for years, with generic versions from Natco, Torrent Pharma, MSN Labs and Eris Lifesciences earlier restrained by courts, after a suit by the multinational firm. Many companies had entered the market at risk and were under constant threat of legal challenge. “Companies are now free to launch, with more players expected to follow, bringing down the price of the therapy further,” an industry expert told TOI.
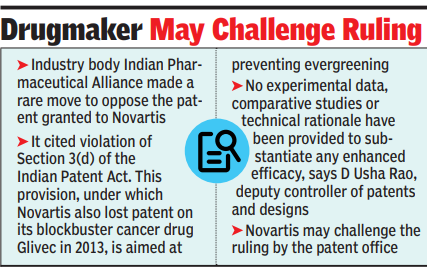
Further, highlighting the drug’s importance is the fact that industry body Indian Pharmaceutical Alliance made a rare move of opposing the patent, along with two firms IPCA and Micro Labs at the post-grant stage, citing a violation of Section 3(d) of the Indian Patent Act. This provision – under which Novartis also lost the patent on its blockbuster cancer drug Glivec in 2013 – is aimed at preventing “evergreening” and has become rare in the industry, being invoked after a long gap.Vymada, sold internationally as Entresto (a combination of sacubitril and valsartan), is a popular hypertension drug, and one of the biggest breadwinners for the Swiss company, having registered $7.8 billion in sales last year.D Usha Rao, deputy controller of patents and designs said in an order dated Sept 12, accessed by TOI, “The patentee has failed to disclose any demonstrated advantages or technical advancement of the claimed supramolecular complex over the combination already disclosed in D1 (closest prior art, patentee’s own earlier application). No experimental data, comparative studies or technical rationale have been provided to substantiate any enhanced efficacy. Further no improved therapeutic efficacy has been shown”. “I have found that the grounds under Section 25(2)(b) – lack of novelty, 25(2)(c) – prior claiming, 25(2) (e) – lack of inventive step, 25(2) (g) complete specification does not sufficiently and clearly describe the invention. Hence the patent no. 414518 is revoked and the said case is disposed of under section 25(2) of The Patents Act, 1970,” the order said.A questionnaire mailed to Novartis failed to elicit a response. Legal sources said the company may challenge the ruling by the patent office. A patent was granted in Dec 2022 to Novartis on its application filed in 2007 for the ‘supramolecular’ complex of the two compounds. It may be pointed out that the company’s patent on another form of the same drug expired in 2023.“The patentee (Novartis) abstained from the hearing. Therefore based on written submissions of both parties order was passed,” legal sources noted.